SESSION 3 — TECHNOLOGY AND INNOVATION SOLUTIONS
 Chair: Susie Stephens, Senior Director, Oncology & West Coast R&D Business Technology, Pfizer Inc.
Chair: Susie Stephens, Senior Director, Oncology & West Coast R&D Business Technology, Pfizer Inc.
Susie Stephens is responsible for R&D IT for the West Coast for Pfizer. As such she provides support for Oncology, Vaccines, Rinat, San Diego and San Francisco Centers for Therapeutic Innovation, and Partner Lines. She is responsible for strategy and delivery of IT services, products, and processes to achieve successful outcomes for R&D.
Susie has cross-disciplinary experience in informatics, science and business from leading pharmaceutical and IT companies. Prior to joining Pfizer, Susie was Head of In Silico Immunology, Janssen Pharmaceutical Research and Development, where she had overall responsibility for in silico science for the Immunology Therapeutic Area. She has also worked for Eli Lilly where she was responsible for Open Innovation for Research IT, Oracle where she created and guided the implementation of their product development strategy for the database for the life sciences, and Sun Microsystems where she was market segment manager for the life science.
Susie has a PhD in Physiology from the University of Exeter, UK; post-doctoral experience in Molecular Biology from the University of Manchester, UK; and is an alumnus of Harvard Business School.
 David Anstey, Global Head LifeSciences, YarcData
David Anstey, Global Head LifeSciences, YarcData
David Anstey is the Global Head of Life Sciences at YarcData, offering an in-depth knowledge of the Pharmaceutical and Biotechnology industries. Dave is responsible for developing and managing the business strategy to deliver YarcData’s solutions within the Life Science markets.
Dave joined YarcData from IDBS where he was Director of Healthcare, North America. Over the past 11 years at IDBS, Dave focused on growing IDBS’ account base and revenue through direct selling and sales management roles. Dave has close to 20 years of experience “selling science” and held key sales roles with Molecular Devices Corp. and Fisher Scientific Canada Ltd prior to IDBS.
Dave holds a BSc in Biology from Carleton University, Ottawa, and an MBA from the University of Ottawa focusing on business strategy and international business.
 Ted Slater, Sr. Solutions Architect, Life Sciences, YarcData
Ted Slater, Sr. Solutions Architect, Life Sciences, YarcData
Ted Slater had recently joined YarcData. Prior to this, he was CTO, OpenBEL Consortium at Selventa, Inc., and Executive Director at Broad Reach Strategic Advising LLC. He is an expert in the application of knowledge- and semantics-based methods to pharmaceutical R&D. He holds an MA in Molecular Biology from the University of California, Riverside, and an MS in Computer Science from New Mexico State University.
Find New Value in Existing Data Using an Enabling Technology for Data Discovery Resulting in Rapid Hypothesis Testing
Big data graph analytics at scale, and in near-real time hypothesis testing can help life sciences organizations take full advantage of their existing data by enabling the capture and exploration of relationships between data. This talk will discuss how large-scale graph analytics approaches can help researchers move beyond the rigid limitations of traditional search to support rapid iterative questioning of diverse data sets. Scalable graph analytics can bring data together in new ways that enables ground breaking discoveries and identifies new targets and opportunities in oncology, neurobiology and other disease areas.
 Dan Housman, Chief Technology Officer, Recombinant By Deloitte and Deloitte Health Informatics
Dan Housman, Chief Technology Officer, Recombinant By Deloitte and Deloitte Health Informatics
Dan is a software veteran with a demonstrated track record of providing valuable and innovative decision support systems to large, complex organizations. Dan leads Recombinant By Deloitte’s strategy, product planning, and development, and is very active in Recombinant By Deloitte client engagements with a focus on translational research, bioinformatics and innovative approaches to data capture, analysis, and reporting for clinical quality and performance improvement. He has conceived and delivered a wide range of effective web-based analytical solutions including clinical performance dashboards, analytic and reporting solutions for performance metrics.
 Raveen Sharma, Subject Matter Advisor, Recombinant By Deloitte and Deloitte Health Informatics
Raveen Sharma, Subject Matter Advisor, Recombinant By Deloitte and Deloitte Health Informatics
Raveen is currently leading the client relationship management function at Recombinant by Deloitte. He has over 20 years of experience in implementing Business Intelligence, Data Warehouse, and Healthcare & Life Sciences Informatics solutions. Raveen has led projects and managed client relationships with academic medical centers, healthcare providers, and pharmaceutical organizations. Projects typically involved data capture, integration of phenotypic and ‘omic data, data preprocessing (normalization, conditioning), analytics (bioinformatics) and delivery to researchers and clinical investigators. Raveen is also active in developing and growing the tranSMART community and has established collaborations for precompetitive technology sharing between several major pharmaceutical organizations.
The Race for Real World Data: Outcomes Data, Analytics, and Convergence with Translational Research and Drug Safety
A solution to bring real world healthcare insights to help transform health outcomes for life science customers.
With healthcare costs on the rise and a movement from volume to value of care occurring, life science companies, providers, and payers require affordable access to data and insights capable of informing outcomes-based decisions. Deloitte’s product suite can help address some of these most pressing issues in healthcare today.
Deloitte and Intermountain Healthcare have established a collaboration that brings together Deloitte’s leading-class professional services and informatics capabilities with Intermountain’s access to patients and pioneering experiences in data-driven care. This collaboration will provide market-leading health analytics solutions that will enable providers and manufacturers to unlock the power of big data, improve quality, and drive efficiencies in the delivery of care and the development of new therapies.
 Christopher Bouton, CEO, Entagen
Christopher Bouton, CEO, Entagen
Dr. Bouton received his BA in Neuroscience (Magna Cum Laude) from Amherst College in 1996 and his Ph.D in Molecular Neurobiology from Johns Hopkins University in 2001. Dr. Bouton is the CEO of Entagen (http://www.entagen.com), a software company founded in 2008 that provides innovative products including Extera and TripleMap. Entagen’s technologies have won numerous awards including the “Innovative Technology of the Year Award for Big Data” from the Massachusetts Technology Leadership Council in 2012 and Entagen was recognized as a Gartner “Cool Vendor” in the Life Sciences in 2013. Prior to his role as the CEO of Entagen Dr. Bouton worked as a computational biologist at LION Bioscience Research Inc. and Aveo Pharmaceuticals from 2001 and 2004, leading the microarray data analysis functions at both companies. In 2004 he accepted the position of Head of Integrative Data Mining for Pfizer and led a group of Ph.D. level scientists conducting research in the areas of computational biology and large-scale ‘omics data analysis. While at Pfizer, Dr. Bouton conceived of and implemented an organization-wide wiki called Pfizerpedia for which he won the prestigious 2007 William E. Upjohn Award in Innovation. Dr. Bouton is an author on over a dozen scientific papers and book chapters and his work has been covered in a number of industry news articles.
Entagen, founded in 2008, provides software and services for “Big Data” environments such as life sciences and healthcare organizations. Entagen’s flagship products are “TripleMap”, a big data analytics application and “Extera”, Entagen’s proprietary Semantic Data Core (SDC) technology. With these technologies Entagen tackles complex Big Data integration and analytics for its clients with a productized software solution that can be securely deployed behind a firewall or in the cloud.
Extera is the dynamic, high-performance semantic data core that bridges the divide between structured and unstructured sources within an organization, allowing for derivation of full value from an organization’s wealth of information and insights. Extera administrators build aggregated representations of entities (e.g.protein targets, compounds, diseases, pathways), their meta-data properties and their associations by connecting to internal and external Big Data sources. This process allows for the creation of a massive interconnected data graph which is continuously updated as it aggregates data from across an organization’s sources such as relational databases, live XML feeds, RDF data sources, Sharepoint TeamSites and literature sources.
TripleMap allows users to constantly scan, create and share structured knowledge maps, thereby connecting the dots in big data and identifying unexpected associations which can drive better hypothesis generation and increase efficiency. Users are dynamically prompted with entities related to their search results, allowing them to explore and visualize the associated information space around their search terms. Knowledge maps also allow users to see who else within their organization has worked on the same entities in the past and to receive alerts to new information as soon as its available from anywhere internal or external to an organization.
Entagen_Overview
Entagen_Overview
 Steve Gun, VP Operations, LifeSciences, Humedica
Steve Gun, VP Operations, LifeSciences, Humedica
Steve serves as Vice President of Life Sciences Operations at Humedica and is responsible for defining, aligning and delivering analyses stemming from partnerships with life sciences clients as well as new product development. Steve brings an array of consulting, analytic and forecasting experience to his role. Prior to Humedica, Steve was Vice President, Strategic Consulting in the healthcare division of Epsilon. Prior to Epsilon, Steve led the consulting and product management divisions of Verispan, L.L.C. for seven years. Steve began his career in forecasting at M/A/R/C Group as a New Product Analyst, after completing his MBA at Emory University and his BA at Middlebury College.
 Mike Sanky, Account Manager, Humedica
Mike Sanky, Account Manager, Humedica
Throughout his career, Mike has specialized in leveraging Big Data solutions to drive health care strategy. Prior to joining Humedica, Mike was a Consultant at the Amundsen Group, where he worked extensively with longitudinal patient data to better understand patient behavior, quality of access, and drivers of product performance. His experience in the biopharmaceutical industry began with the Center for Pricing and Reimbursement at United BioSource Corporation. Mike received his MS in Marketing Analytics from the McCallum School of Business at Bentley University (where he published a health care intervention study in the Journal of Medical Marketing) and his BS from the Walsh School of Foreign Service at Georgetown University.
Electronic Health Record Analytics
Humedica is the foremost clinical intelligence company that provides private cloud-based business solutions to the health care industry. Humedica NorthStar™ leverages Spotfire to deliver Humedica’s high-quality Electronic Health Record (EHR) data in a userfriendly, flexible format. The platform is designed to foster the discovery of areas of opportunity within highly detailed clinical segments, to identify the triggers for specific brand choices within those segments, and to refine marketing approaches.
In our presentation, we will utilize NorthStar™ to demonstrate unique insights stemming from clinical data and physician notes information captured in EHR.
Clinical Data
EHR contains a wealth of real-world clinical data including lab results (like A1C) and vital signs (like BMI). In the Diabetes market, A1C and BMI are very important factors that determine prescribing decisions.
The graph below shows class-level market share by each patient’s pre-Rx A1C cohort (the most recent A1C that is within 90 days of the Written Prescription). As patients have more severe A1Cs, we see that they are more likely to get prescribed Insulin. (Figure 1)
In addition to A1Cs, we now layer information related to BMI in the graph below (Figure 2). GLP-1s are frequently prescribed to obese patients because of a weight-loss side effect, making its market niche clear.
Physician Notes
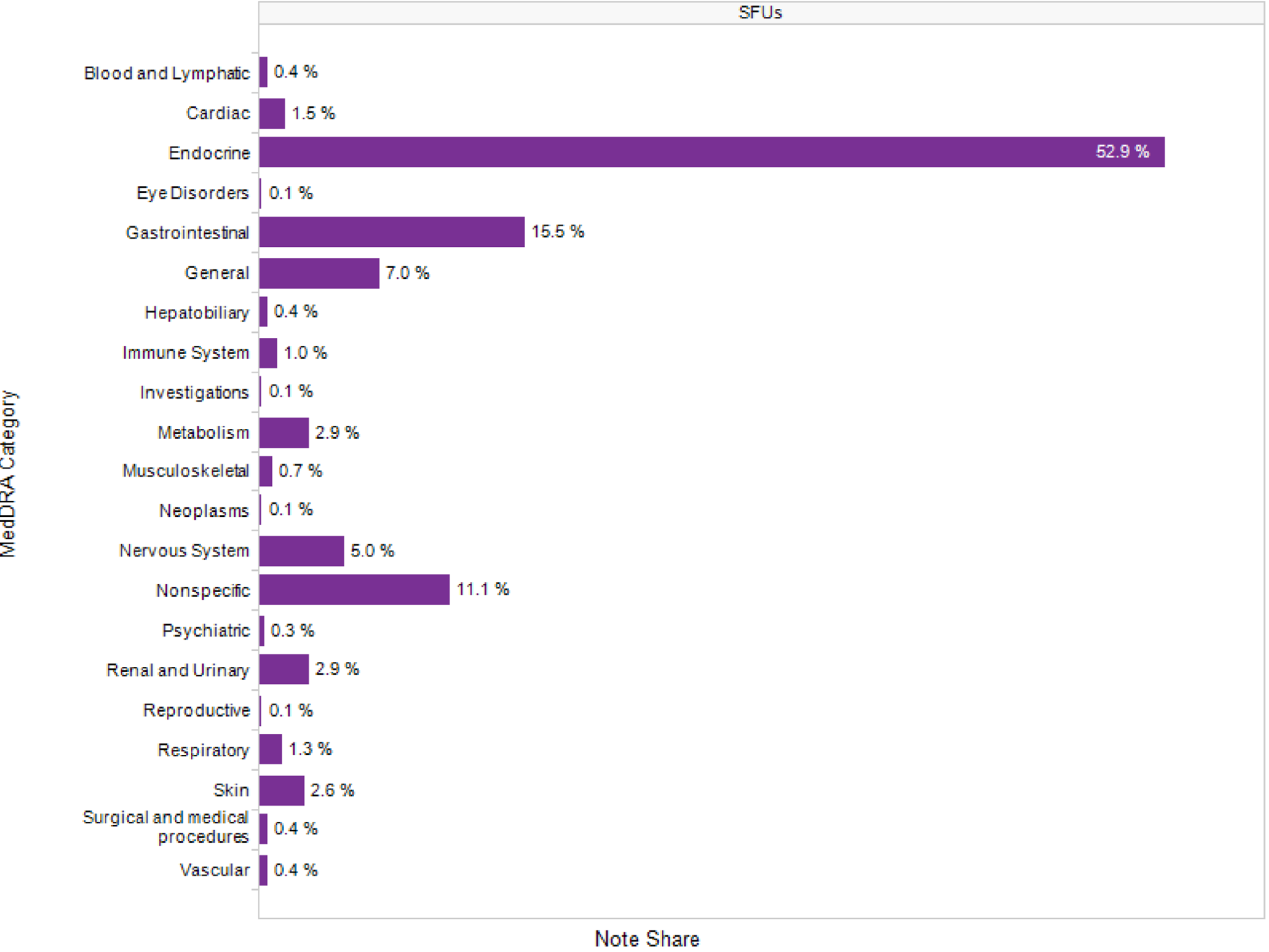
In addition to rich clinical data, physician notes offer another unique attribute in EHR data. The graph below (Figure 3) shows discontinuation rationale by Signs, Diseases, and Symptoms that are mapped to the Medical Dictionary for Regulatory Activities (MedDRA). In the example below, when a physician notes a clinical reason for a sulfonylurea discontinuation, more than half of the time it is due to endocrine reasons (the majority of which are hypoglycemia). Brand managers looking to increase Line 2 post-metformin market share should refine their messaging to increase awareness around patients who are not good candidates for sulfonylureas because of hypoglycemia risk.
http://www.humedica.com
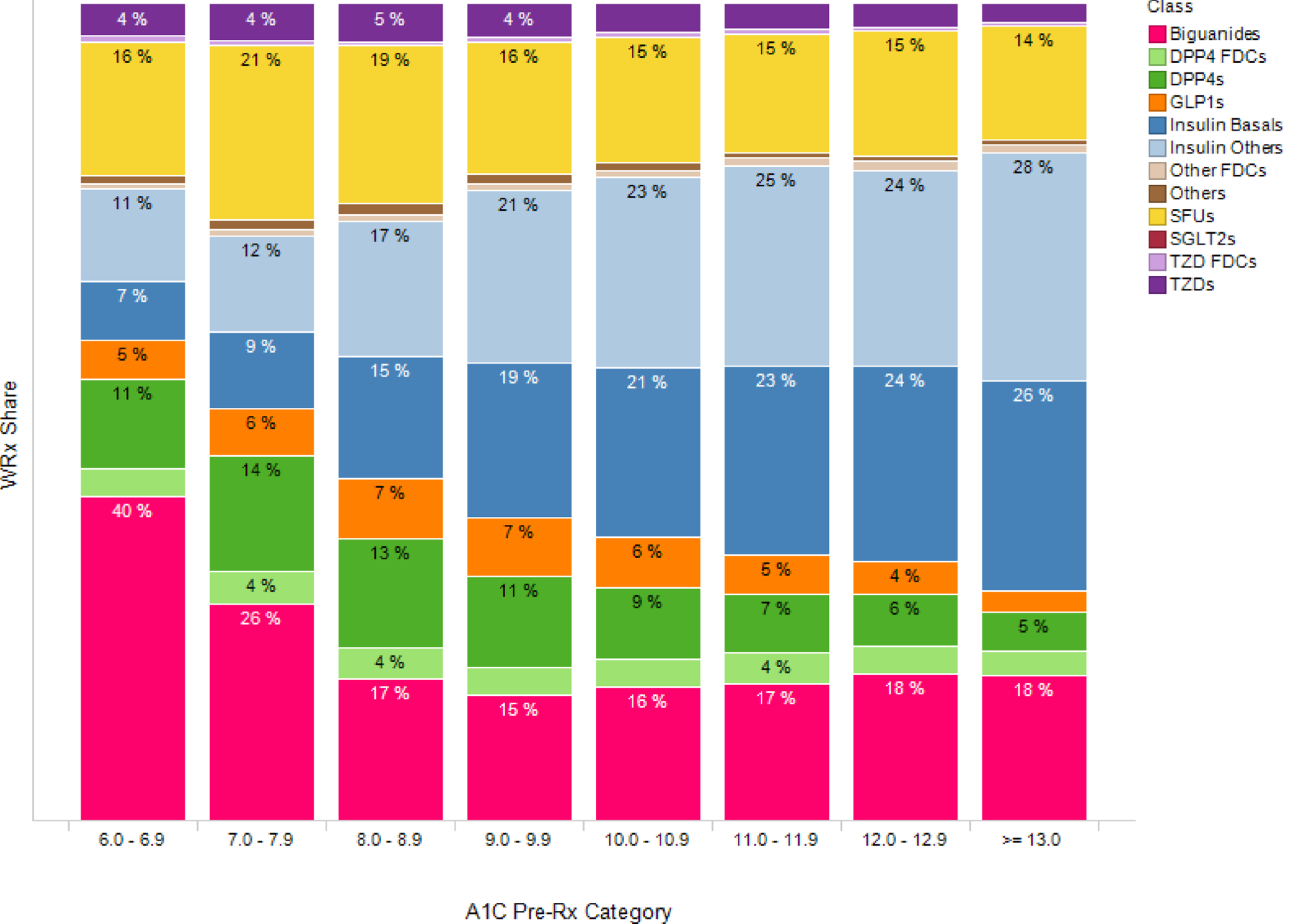 Fig 1
Fig 1
Class-Level Market Share by Pre-Rx A1C (Jan 2010 – May 2013)
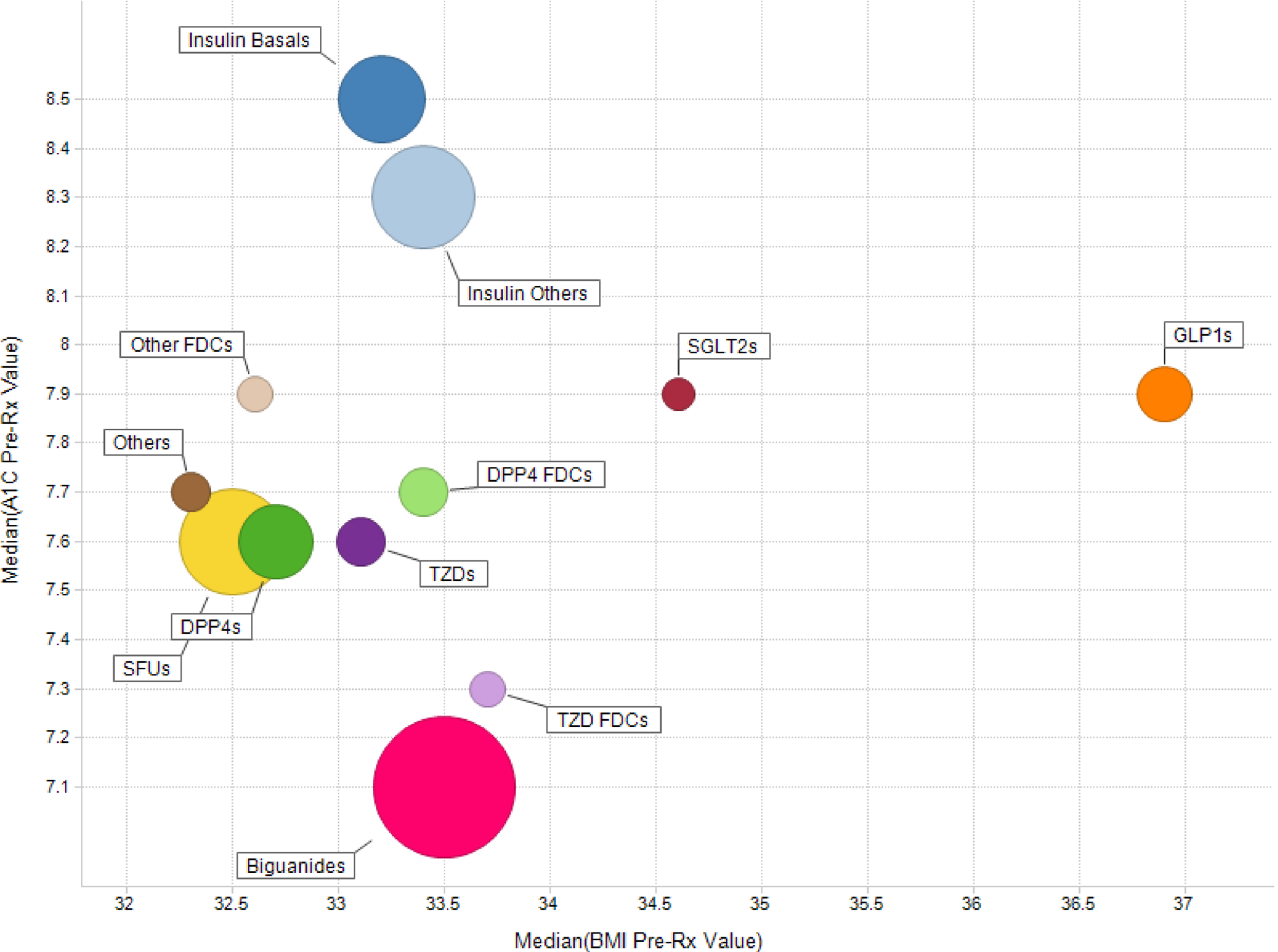 Fig 2
Fig 2
Median Pre-Rx A1C and BMI by Class (Jan 2010 – May 2013)
Physician Considerations for Sulfonylurea Discontinuation (Jan 2010 – May 2013)
Humedica-NorthStar
Humedica-NorthStar
 Noah Zimmerman, VP Data Science, Co-Founder, Kyron
Noah Zimmerman, VP Data Science, Co-Founder, Kyron
Noah Zimmerman has a background in computer science with training in statistics, immunology and medicine. Prior to Kyron, he was a Senior Data Scientist at Pivotal where he helped build the data science practice for the healthcare & life-science vertical.
He completed his doctoral work in Biomedical Informatics at Stanford, and was a member of the founding team of 2 startups in the healthcare/life-science space.
In addition to his data science duties, Noah is an instructor for a course he co-created at the Stanford d.school that explores the intersection of science and design.
Patients Like Mine: A Scalable Model for Medical Discovery
The current paradigm of evidence-based medicine through prospective clinical trials does not scale. Among Americans aged 60 and over, more than 75% use two or more prescription drugs and 37% use five or more. A half-million trials would be required to test all of the possible 2-drug combinations that close to 40 million Americans are currently taking, and 10 million more trials to test all of the 5-drug combinations.
The volume of potential interactions and population-level heterogeneity (ethnicity, co-morbidities, environment, etc.) make the problem intractable. Therefore, we need new techniques for (a) post-market drug surveillance and (b) data-driven prioritization of follow-up studies. These methods will complement the prospective clinical trial, providing a view into drug safety and efficacy experiments in the wild.
Every day, clinical decision-making in the wild results in tens of thousands of micro-experiments. In aggregate, these micro-experiments enable learning of real-world outcomes. Analysis of Electronic medical records (EMR) enables systematic examination of factors leading to differential outcomes in highly targeted patient populations.
At Kyron, we are developing advanced tools to process EMR data for rapid search, query and data mining. Our core technology transforms the textual notes from a patient record into a structured patient profile consisting of concepts from medical ontologies, which allows search and query of patient records at varying levels of granularity along the concept hierarchies.
These patient profiles have been used as a substrate for multiple applications including drug safety surveillance and off-label drug-use. In some state of the art studies, the technology underlying Kyron could detect major adverse events associated with 6 of the last 9 major drug recalls in the last decade ~2 years before the recall occurred, could detect roughly 80% of known drug-drug interactions, and demonstrated the safety of a highly effective drug for peripheral vascular disease.